Tirzepatide Treatment in Westminster, Colorado
What is Tirzepatide Treatment?
Tirzepatide is a novel medication designed to aid in the management of type 2 diabetes. It is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, meaning it targets two key hormones involved in blood sugar regulation. By mimicking the action of these hormones, Tirzepatide helps to lower blood glucose levels, improve insulin sensitivity, and potentially facilitate weight loss. This innovative treatment offers a new approach for individuals struggling to manage their diabetes with traditional therapies.What are the Benefits of Tirzepatide Treatment in Westminster, Colorado?
The benefits of Tirzepatide treatment are multifaceted, addressing various aspects of diabetes management and overall health:
Effective Blood Sugar Control
Tirzepatide has been shown to significantly reduce HbA1c levels, a key marker of long-term blood glucose control. This helps in maintaining stable blood sugar levels, reducing the risk of diabetes-related complications.
Weight Loss
Many individuals with type 2 diabetes struggle with weight management. Tirzepatide has demonstrated a potential for weight loss, which can further enhance blood sugar control and reduce the burden on the cardiovascular system.
Improved Insulin Sensitivity
By enhancing the body’s response to insulin, Tirzepatide helps in better utilization of glucose, reducing the need for additional insulin therapy and minimizing the risk of hypoglycemia.
Cardiovascular Benefits
Research indicates that Tirzepatide may offer cardiovascular benefits, such as reducing the risk of heart disease, which is a common concern for individuals with type 2 diabetes.
Convenience
Tirzepatide is administered once weekly, offering a convenient option for those who prefer fewer injections compared to daily insulin or other diabetes medications.
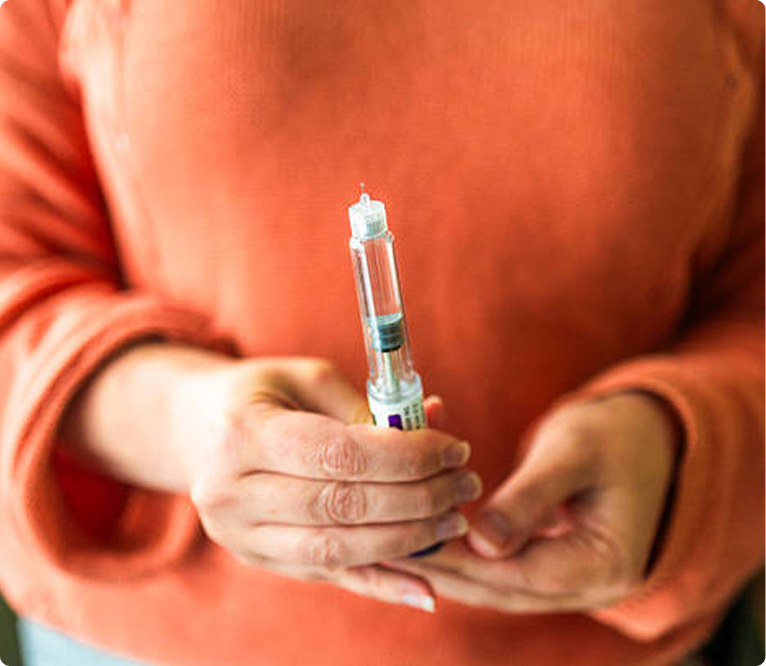

Who is Tirzepatide Treatment in Westminster, Colorado Right For?
Tirzepatide treatment is suitable for a wide range of individuals with type 2 diabetes, particularly those who:
However, it is essential to consult with a healthcare provider to determine if Tirzepatide is the right choice based on individual health needs and medical history.
What Conditions Can Tirzepatide Treatment in Westminster, Colorado Treat?
Tirzepatide is primarily designed to treat type 2 diabetes, but its benefits extend to several related conditions:
Type 2 Diabetes
The primary indication for Tirzepatide is the management of type 2 diabetes, helping to lower blood sugar levels and improve overall glycemic control.
Obesity
Given its potential for weight loss, Tirzepatide can be beneficial for individuals with type 2 diabetes who are also struggling with obesity.
Cardiovascular Risk
For patients with type 2 diabetes and existing cardiovascular risk factors, Tirzepatide may provide additional protection against heart disease.
Insulin Resistance
By improving insulin sensitivity, Tirzepatide helps in managing conditions associated with insulin resistance, such as metabolic syndrome.
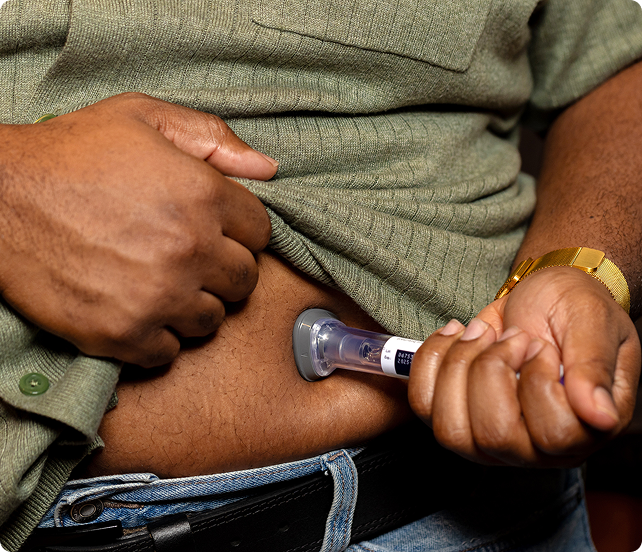
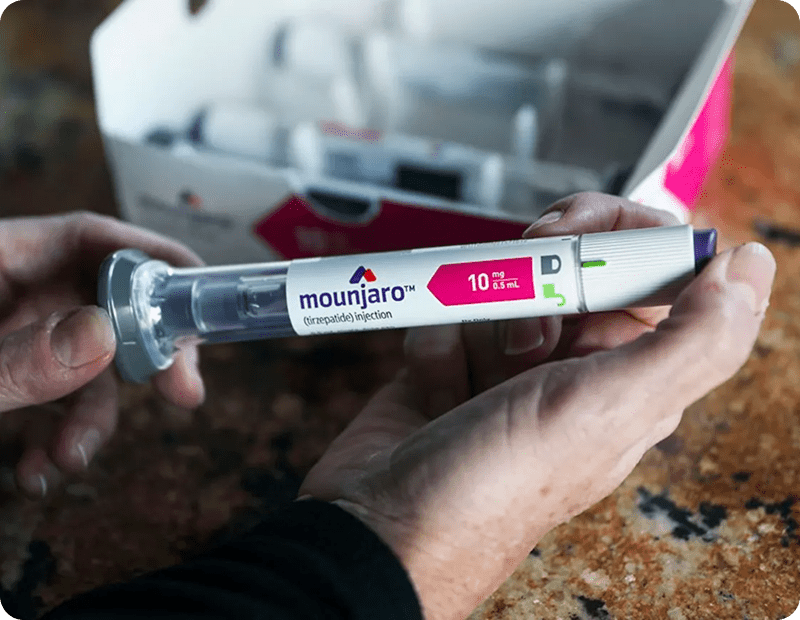
What Should I Try at Home Before Seeing a Provider for Tirzepatide Treatment in Westminster, Colorado?
Before considering Tirzepatide treatment, there are several lifestyle and dietary adjustments you can try at home to manage type 2 diabetes:
Dietary Changes
Regular Exercise
Monitoring Blood Sugar
Stress Management
Hydration
Our Process for Determining if You Are a Candidate for Tirzepatide Treatment in Westminster, Colorado
At Awaken IV, we prioritize personalized care and thorough evaluations to determine the best treatment options for our clients. Our process for assessing candidacy for Tirzepatide treatment involves several key steps:
Initial Consultation
During your initial consultation, our healthcare providers will conduct a comprehensive review of your medical history, current medications, and overall health status. This helps us understand your specific needs and treatment goals.
Blood Tests and Assessments
We may recommend blood tests to evaluate your HbA1c levels, kidney function, and other relevant markers. These tests provide valuable insights into your diabetes management and overall health.
Personalized Treatment Plan
Based on the consultation and test results, our team will develop a personalized treatment plan tailored to your needs. If Tirzepatide is deemed appropriate, we will provide detailed instructions on how to use the medication and what to expect.
Ongoing Monitoring and Support
Our commitment to your health doesn’t end with the initial treatment plan. We offer ongoing monitoring and support to ensure the effectiveness of the treatment and make any necessary adjustments. Regular follow-up appointments and blood tests will help us track your progress and address any concerns.


Conclusion
Tirzepatide treatment represents a promising advancement in the management of type 2 diabetes, offering benefits that extend beyond blood sugar control to include weight loss and potential cardiovascular protection. At Awaken IV, we are dedicated to providing personalized, high-quality care to help our clients achieve their health and wellness goals. If you are struggling with type 2 diabetes and looking for a comprehensive treatment approach, our team is here to support you every step of the way. By combining innovative treatments like Tirzepatide with lifestyle modifications and ongoing support, we aim to empower our clients to take control of their health and live their best lives.
Treatment Locations
- Arvada, CO
- Aurora, CO
- Boulder, CO
- Brighton, CO
- Broomfield, CO
- Castle Pines, CO
- Castle Rock, CO
- Centennial, CO
- Cherry Hills Village, CO
- Colorado Springs, CO
- Denver, CO
- Englewood, CO
- Erie, CO
- Fort Collins, CO
- Golden, CO
- Greeley, CO
- Greenwood Village, CO
- Highlands Ranch, CO
- Lafayette, CO
- Lakewood, CO
- Littleton, CO
- Lone Tree, CO
- Longmont, CO
- Louisville, CO
- Loveland, CO
- Northglenn, CO
- Parker, CO
- Superior, CO
- Thornton, CO
- Westminster, CO
